Paralyzed rats walk again after experimental treatment
Cortical plasticity helps recovery of weight-supported hindlimb stepping
Adult spinal cord injuries often result in permanent loss of mobility because the nervous system can’t completely heal spinal connections. Researchers have focused on two approaches to recovering movement: healing the injury, or bypassing it to reconnect motor circuits above and below. Both approaches assume that the adult nervous system can’t develop its own way to bypass the injury.
Karen Moxon, a professor of biomedical and mechanical engineering at UC Davis, questioned that assumption and in a new paper published in eLife, shows that a combined therapeutic regimen of drugs and physical therapy promoted cortical reorganization that bypassed the injury, allowing paralyzed rats to walk again.
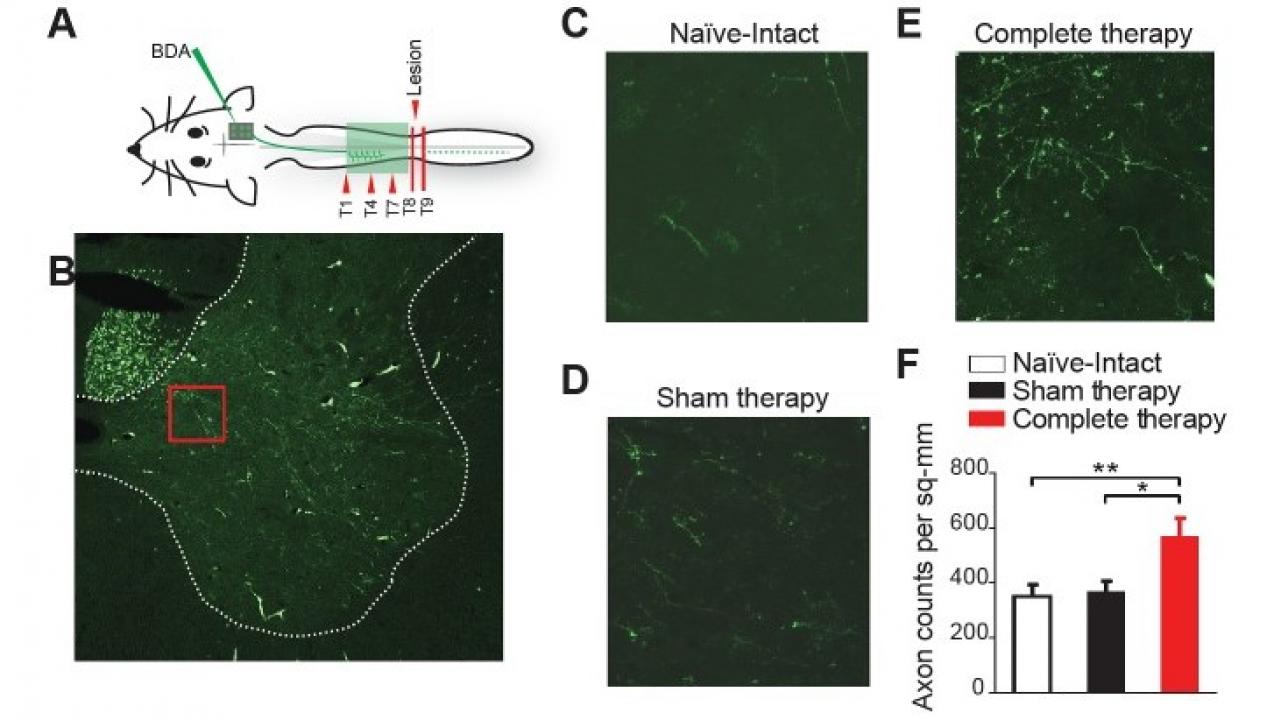
Moxon’s group treated rats with severed spinal cords using drugs that activate serotonin receptors in the brain, particularly the 5-HT receptors, and physical therapy that targeted either movement below the injury (partial therapy), or movement both above and below (complete therapy). After 12 weeks, animals that had received complete therapy could walk significantly better than the rats that had received partial therapy or no therapy. In fact, three individuals that had received complete therapy were able to perform greater than 40% of weight supported step cycles on a treadmill, and one animal went as high as 56%.
Tests on the brains of rats which regained movement revealed that the motor cortex had reorganized itself to bypass the injured region of the spinal cord. Corticospinal fibers from the reorganized motor cortex sprouted into the cord above the lesion after therapy, forming an entirely new motor pathway.
Further investigation suggested that involvement of both fore and hindlimbs in the physical therapy encouraged more neurons to grow than in either partial or no therapy.
The results of this study suggest that the treated rats learned to activate the muscles in their trunks to reduce the load on the hindlimbs while balancing on the forelimbs. This allowed neural circuits in the spinal cord that are responsible for producing stepping motions below the level of the lesion to be activated, resulting in weight-supported hindlimb stepping.
Although the study presents a tantalizing possibility for human motor recovery, Moxon warns that these results might only apply to quadrupedal locomotion. Humans, who walk bipedally, might not obtain the same level of benefit. However, the study lends strong support to the idea that whether in humans or rats, well-designed treatment plans that combine drugs with physical therapy can have synergistic effects on cortical plasticity and recovery.
Cortex-dependent recovery of unassisted hindlimb locomotion after complete spinal cord injury in adult rats. Anitha Manohar, Guglielmo Foffani, Patrick D Ganzer, John R Bethea, Karen A Moxon. eLife 2017;6:e23532 DOI: 10.7554/eLife.23532
