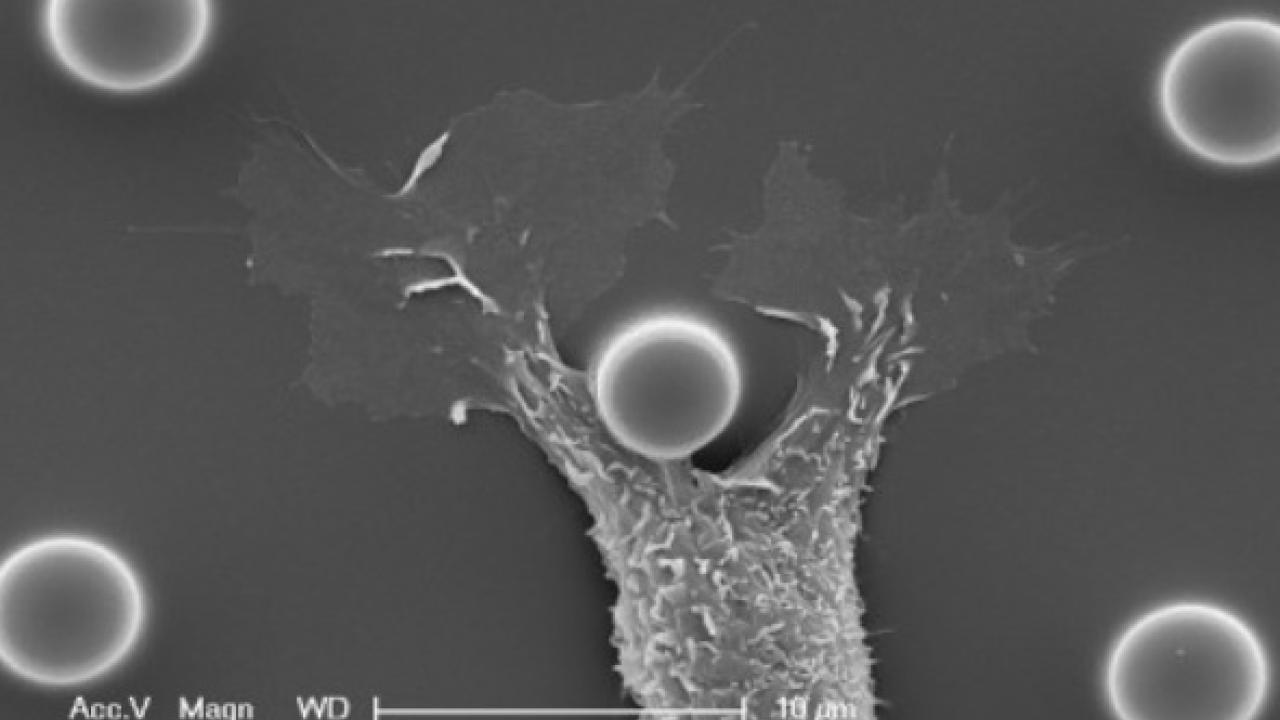
A Rouge Mirror Test for Individual Cells
The rouge mirror test determines babies’ self-recognition level. With a spot of makeup on their face, one-year-old babies will recognize the self in the mirror and try to remove the rouge spot. Aided by sight and touch, self-recognition by an organism may be apparent, but does self-recognition also occur at the cellular level? We already know that immune cells can readily identify foreign pathogens through recognition of cell surface receptors. But what about a set of identical cells with identical surface chemistry that form tissue and organs, do they distinguish self from neighboring cells?
To elucidate this topic, a new paper by Soichiro Yamada, a professor of biomedical engineering at UC Davis, addresses a simple question, “What happens when single epithelial cells contact their own membrane versus the membrane of neighboring cells?” The paper was published in the November 19 issue of “Proceedings of the National Academy of Sciences”.
Dr. Yamada and his postdoctoral fellow Dr. Grant Sumida designed an array of micro-pillars as barriers, promoting cells to extend their membranes around individual pillars and ultimately contact their own membrane (see Figure). Interestingly, normal epithelial and endothelial cells passed this cell-level rouge test, but fibroblasts and some cancer cells failed. In other words, fibroblasts and some cancer cells develop cell junctions when they interact with their own membrane, similar to how they interact with neighboring cells. In contrast, normal epithelial cells and endothelial cells do not establish mature cell junctions; rather, self-contacting membranes dissolve and fuse.
This novel observation suggests that normal epithelial cells and endothelial cells have an ability to discriminate self and neighboring membranes, despite the identical surface chemistry. Additionally, the fibroblast-like behavior of epithelial-derived cancer cells in this cell-level rouge test suggests that self-recognition defects may trigger the development of aggressive cancers.
As to the molecular details, Dr. Sumida explains, “we know that some sort of mechanical signal is important in this self-recognition” citing their findings on the roles of E-cadherin, a cell-cell adhesion protein, and ROCK, a regulator of cellular contractility, in self-contact induced membrane fusion. “But, we still do not know the precise mechanisms by which individual cells discriminate self from neighboring cells”, says Dr. Sumida.
Dr. Yamada praises NIH for funding these types of high-risk, high-reward projects. This project is funded through NIH’s unique funding program called Exceptional, Unconventional Research Enabling Knowledge Acceleration (EUREKA) program, and Dr. Yamada says “without EUREKA funding, we would not have discovered cell-level self-recognition for who knows how long”. The team is currently investigating the molecular details of cell self-recognition, and how mechanical force (strong vs. weak touching) may be a discriminating factor for the cell-level rouge mirror test.
–by Grant Sumida and Soichiro Yamada
